Next milestone in the treatment of liver tumors and acute and chronic liver diseases
The results of a study led by the DKTK partner site in Tübingen give reason to hope that a newly developed drug could start a new era in oncological liver surgery and liver transplantation. The drug could also have the potential to significantly improve the treatment of acute and chronic liver diseases. The results of the study have now been published in the renowned scientific journal Cell.
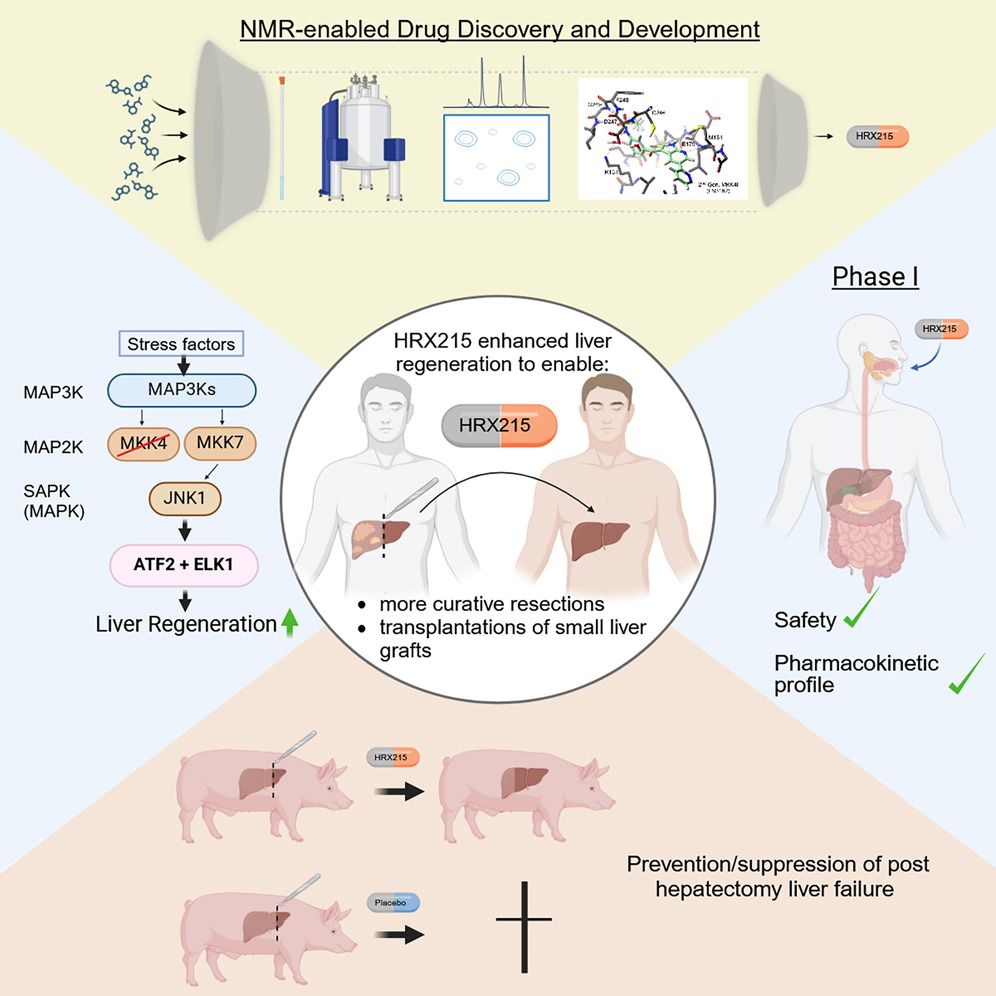
The drug candidate "HRX-215" is a so-called MKK4 inhibitor, which inhibits the MKK4 protein found in liver cells and increases the regeneration of liver cells. The preclinical and phase I trials, led by Prof. Dr. Lars Zender (Medical Director, Department of Medical Oncology and Pneumology, University Hospital Tübingen and scientist at the German Consortium for Translational Cancer Research (DKTK), partner site Tübingen), were made possible by a collaboration between scientists from Tübingen, the Tübingen start-up HepaRegeniX and researchers from the Mayo Clinic (USA).
Therein, preclinical studies in mouse and pig models have shown that the increased liver regeneration caused by the inhibitor HRX-215 allows liver surgery that was previously not possible. Until now, it has not been possible to surgically remove all of the affected tissue in advanced liver tumors, for example, as this would lead to liver failure in the remaining liver. The extended partial liver removal made possible by HRX-215 could lead to the complete removal of advanced liver tumors. In addition, the active substance could be able to provide more people with a life-saving liver transplant. A phase I study in 48 healthy volunteers showed excellent tolerability of the drug.
Liver disease as a growing health problem
Liver disease is a global health problem and is responsible for more than two million deaths per year. The number of deaths has risen by 50 percent in recent decades and is expected to double in the next 20 years. Although the liver is one of the few organs that can regenerate itself, this property has its limits. Particularly in chronic and acute liver diseases or after the surgical removal of a large part of the organ, the liver cells can no longer regenerate sufficiently. The consequence is often fatal liver failure. Even today, the last resort for end-stage liver disease is a liver transplant. However, due to the shortage of donor organs, only ten percent of all affected patients can receive a life-saving liver transplant.
Clinical results give cause for hope
As there were previously no drugs that could increase the regeneration of a damaged liver, the published results with the new active ingredient HRX-215 are a milestone: "The positive results in terms of tolerability confirm our intention to be able to offer a drug in the future that has the potential to revolutionize the treatment of acute liver diseases," emphasizes Dr. Wolfgang Albrecht, CEO of the Tübingen-based start-up company HepaRegeniX. The current results and the spin-off of HepaRegeniX were largely made possible by Prof. Zender's groundbreaking discovery in 2013. "By inhibiting the kinase MKK4, the self-healing function of a damaged liver can be triggered," says first author Stefan Zwirner, summarizing the findings.
Possible solution to organ donation shortage?
"HRX-215 would not only be an urgently needed treatment option in the surgical removal of liver tumors, but could also help to overcome the major problem of organ shortage in the field of liver transplantation," Prof. Zender points out the possible applications. Live transplants from the smaller left part of the liver of a healthy donor would be a solution, as the removal of the liver poses only a low health risk for healthy donors. However, this part of the liver is often too small to take over the function of the liver removed from the recipient. "Due to the rapid enhancement of liver regeneration mediated by HRX-215, we assume that HRX-215 treatment would enable the safe transplantation of small left liver lobes in adults," continues Prof. Zender. However, further clinical studies must show this.
Academic center for drug development
"When we started developing an MKK4 inhibitor in 2017, none of us could have imagined that we would be able to start the first clinical trial just four years later and present the first results of the Phase I trial three years later," adds Prof. Stefan Laufer, whose research group carried out the medicinal chemistry work on the first MKK4 inhibitor. The development of MKK4 inhibitors is a success story and underlines the leading national and international role of the Tübingen site in the field of drug development and optimization. With the Cluster of Excellence iFIT (Image Guided and Functionally Instructed Tumor Therapies) and the Tübingen Center for Academic Drug Discovery & Development (TüCAD2), whose founder and spokesperson is Prof. Laufer, the university has a unique platform for the identification, validation and development of innovative drug candidates, which has already brought several candidates to first application in humans.
German-American cooperation
Before the active substance was tested on healthy volunteers in the Phase I study, it was used in animal models (mice and pigs). For the studies in pigs, we collaborated with the Mayo Clinic (USA) with Prof. Dr. Scott. L. Nyberg, an internationally renowned expert in the field of transplantation and regenerative medicine. His team was able to show that the use of HRX-215 increased liver regeneration in the animals and prevented liver failure, even after removal of 85 percent of the organ.
Original publication: First-in-class MKK4 Inhibitors enhance Liver Regeneration and prevent Liver Failure. Zwirner, S., Abu Rmilah, A.A., Klotz, S., Pfaffenroth, B. et al. Cell. 2024 Mar 28;187(7):1666-1684.e26
Source: DKTK

Graphical abstract © https://doi.org/10.1016/j.cell.2024.02.023







